electronic configuration of praseodymium|Complete Electron Configuration for Praseodymium (Pr) : Tagatay Electron configuration: [Xe] 4f 3 6s 2: Density @ 20 o C: 6.77 g/cm 3: Show more, including: Heats, Energies, Oxidation, Reactions, Compounds, Radii, Conductivities. . Praseodymium is used in hybrid car electric .
AXEIA offers affordable housing in the Philippines. Find out more today about our affordable homes in Cavite, Batangas, Laguna, Pampanga and Rizal.
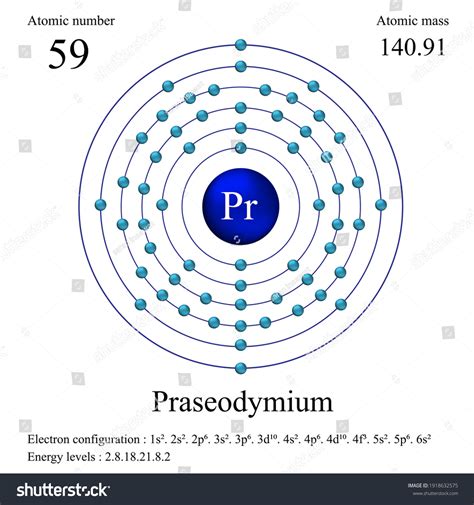
electronic configuration of praseodymium,The arrangement of electrons in praseodymium in specific rules in different orbits and orbitals is called the electron configuration of praseodymium. The electron configuration of praseodymium is [ Xe ] 4f 3 6s 2 , if the electron arrangement is .
electronic configuration of praseodymium Complete Electron Configuration for Praseodymium (Pr)Praseodymium metal tarnishes slowly in air, forming a spalling green oxide layer like iron rust; a centimetre-sized sample of praseodymium metal corrodes completely in about a year. It burns readily at 150 °C to form praseodymium(III,IV) oxide, a nonstoichiometric compound approximating to Pr6O11:These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Atomic numberThe number of protons in an atom. .Electron configuration for praseodymium. The history of Praseodymium. Periodic table history. Identifiers. List of unique identifiers for Praseodymium in various chemical . Electron configuration of Praseodymium is [Xe] 4f3 6s2. Possible oxidation states are +3. Electron Configuration. The periodic table is a tabular display .Electron configuration: [Xe] 4f 3 6s 2: Density @ 20 o C: 6.77 g/cm 3: Show more, including: Heats, Energies, Oxidation, Reactions, Compounds, Radii, Conductivities. . Praseodymium is used in hybrid car electric .
Below is the electronic diagram of the Praseodymium atom Distribution of electrons over energy levels in the Pr atom 1-st level (K): 2 2-st level (L): 8 3-st level (M): 18 4-st level .
The electronic configuration of Praseodymium is [Xe]544f36s2. It is a yellowish metal with a soft and malleable texture. The metal has an atomic number of .
PRASEODYMIUM. TRANSITION ELEMENT: LANTHANIDE. Praseodymium was discovered by Carl Freiherr Auer von Welsbach (AT) in 1885. The origin of the name . List all Pr properties. Praseodymium atoms have 59 electrons and the shell structure is 2.8.18.21.8.2. The ground state electron configuration of ground state gaseous neutral praseodymium is [ Xe ]. .

Orbital diagram. Praseodymium electron configuration. ← Electronic configurations of elements. Pr (Praseodymium) is an element with position number 59 in the periodic table. Located in the VI period. Melting point: 931 ℃. Density: 6.48 g/cm 3 . Electronic configuration of the Praseodymium atom in ascending order of orbital energies: 1s 2 .
Misch metal, used in making cigarette lighters, contains about 5% praseodymium metal. The rare-earth oxides, including Pr 2 O 3 are among the most refractory substances known. Along with other rare earths, it is widely used as a core material for carbon arcs used by the motion picture industry for studio lighting and projection.Electron configuration 4f 3 6s 2: Electrons per shell: 2, 8, 18, 21, 8, 2: Physical properties; . Praseodymium is a chemical element; it has symbol Pr and the atomic number 59. It is the third member of the lanthanide .The electronic configuration of 59 P r (Praseodymium) is? A [54 X e] 4 f 2 5 d 1 6 s 2. B [54 X e] 4 f 1 5 d 2 6 s 2. C [54 X e] 4 f 3 6 s 2. D [54 X e] 4 f 3 5 d 2. Open in App. Solution. Verified by Toppr. . Stable electronic configuration & unstable electronic configuration of Mg are : View Solution. Q5.

In the case of Praseodymium the abbreviated electron configuration is [Xe] 4f3 6s2. Nevertheless, check the complete configuration and other interesting facts about Praseodymium that most people don't know. Praseodymium Overview Praseodymium Complete Electron Configuration 1s2 2s2 2p6 3s2 3p6 4 s2 3 d10 4 .Electron configuration: [Xe] 4f 3 6s 2: Density @ 20 o C: 6.77 g/cm 3: Show more, including: Heats, Energies, Oxidation, Reactions, Compounds, Radii, Conductivities. . Praseodymium is used in hybrid car electric motors and generators, iPods, studio lighting and aircraft engines. Praseodymium colored glass. (photo: .
Praseodymium Atomic Number and Electronic Configuration . Atomic number of Praseodymium is 59. Its electronic configuration is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 3 5d 0 6s 2 or it can be written as [Xe] 4f 3 6s 2. It has 2 electrons in K – shell, 8 electrons in L – shell, 18 electrons in M – shell and 21 electrons .The electronic configuration of Praseodymium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f3 6s2. What is the abbreviated electronic configuration of Praseodymium? The abbreviated electronic configuration of Praseodymium is [Xe] 4f3 6s2. To form abbreviated notation of electronic configuration, the completely filled .Molar volume: 20.8 × 10 -6 m 3 /mol. Protons/Electrons: 59. Neutrons: 82. Shell structure: 2,8,18,21,8,2. Electron configuration: [Xe]6s24f3. Oxidation state: 3. Crystal structure: hexagonal. Praseodymium should be stored under a light mineral oil or sealed in plastic, as it will develop a green coating which, in return, exposes more metal to . Praseodymium is a chemical element that belongs to the 6th period and is the third member of the Lanthanide series. The praseodymium element's symbol is Pr. It is a metal that has long been thought to be one of the rare earth metals. The electronic configuration of parseodymium is: 1s 2 2s 2 2p 6 3s 22 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 .
Electronic configuration [Xe] 4f 3 6s 2: Atomic radius: 239 picometers (van der Waals radius) 1st Ionization energy: 5.464 eV: Electronegativity: 1.13 (Pauling scale) . The last electron in praseodymium enters the f-orbital and hence it is classified as a f-block element on the periodic table.Here, the electron configuration of praseodymium ion is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 2 5s 2 5p 6. This praseodymium ion(Pr 3+) has fifty-nine protons, eighty-two neutrons, and fifty-six electrons.
As is the same for all of the lanthanoids, the chemistry of praseodymium is dominated by the +3 oxidation state — but its electronic configuration of [Xe]4 f3 6 s2 makes it a good candidate for .
The praseodymium element also increases the power of magnets containing it. Electron configuration. The electron configuration of an element describes the arrangement of electrons in the atoms of that element, and be used to predict its chemical properties and reactivity.
Electronic configuration: [Xe] 4f 3 6s 2: Formal oxidation number: +3: Electronegativities: 1.13: Atomic radius / pm: 182: Relative atomic mass: 140.907 66(2) Praseodymium was discovered by Carl Freiherr Auer von Welsbach (AT) in 1885. The origin of the name comes from the Greek words prasios didymos meaning green twin. It is a silvery white .electronic configuration of praseodymiumThis page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. For phosphorus (element 15) as an example, the concise form is [Ne] 3s 2 3p 3.Complete Electron Configuration for Praseodymium (Pr)Electron configuration: [Xe]6s24f3 Oxidation state: 3 Crystal structure: hexagonal. Praseodymium should be stored under a light mineral oil or sealed in plastic, as it will develop a green coating which, in return, exposes more metal to oxidation.
Electron configuration and elemental properties of praseodymium. (Image credit: Greg Robson/Creative Commons, Andrei Marincas Shutterstock) Word origin: Praseodymium comes from the Greek word .
electronic configuration of praseodymium|Complete Electron Configuration for Praseodymium (Pr)
PH0 · Praseodymium»properties of free atoms [WebElements
PH1 · Praseodymium Element Facts / Chemistry
PH2 · Praseodymium Electron Configuration(Explained for Beginners)
PH3 · Praseodymium (Pr)
PH4 · Praseodymium
PH5 · Electron configuration for Praseodymium (element 59). Orbital
PH6 · Complete Electron Configuration for Praseodymium (Pr)